Publications
> 2025
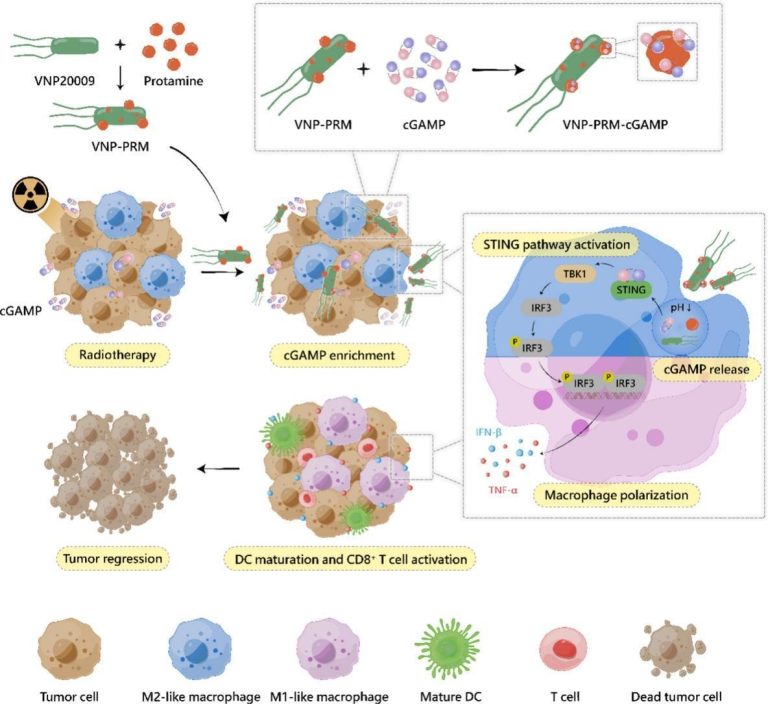
In situ enrichment and delivery of STING agonists by protamine-modified Salmonella for cancer immunotherapy
Abstract
Immunotherapy has emerged as a promising therapeutic strategy for cancer. The activation of the stimulator of interferon genes (STING) pathway promotes the polarization of tumor-associated macrophages (TAMs) towards M1 phenotype, with 2′, 3′-cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) serving as an inherent activator, which significantly accumulates in the extracellular space of the tumor sites following radiotherapy (RT). However, the electronegativity and hydrophilicity of cGAMP prevent it from crossing the cell membrane into TAMs, hampering subsequent immunotherapy efficacy. Here, positively charged protamine-modified Salmonella (VNP20009), called VNP-protamine (VNP-PRM), were prepared to enrich cGAMP and form a composite bacteria-drug delivery system with the capacity to enter TAMs freely. Via electrostatic interactions between the guanidine groups of protamine and the phosphate groups of cGAMP, VNP-PRM stably enriched cGAMP on their surface and neutralized the electronegativity of cGAMP. The uptake efficiency of cGAMP by TAMs was then significantly enhanced by the active delivery of VNP-PRM, whose inherent motility and cellular invasiveness endowed them with increased potential to bump against and enter the macrophages. Subsequently, the intracellular cGAMP synergized with the immunogenicity of the bacteria to activate the STING pathway and drive M1 polarization, thereby boosting tumor eradication. Therefore, antitumor immunotherapy can be optimized through in situ enrichment and delivery of post-RT cGAMP to TAMs by the engineered bacteria.
Read More
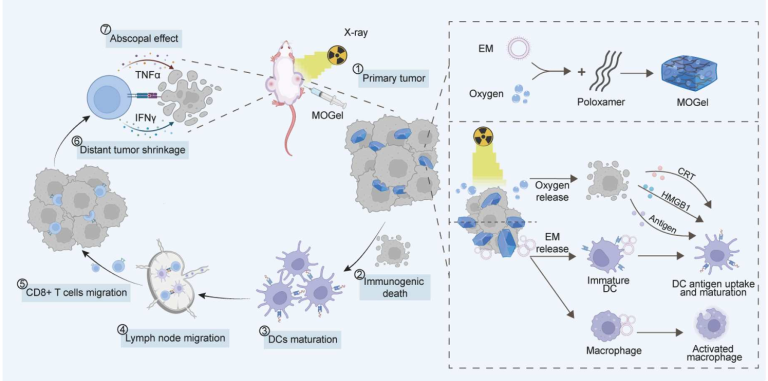
Bacteria outer membrane-based oxygen gels alleviate tumor hypoxia for enhanced systemic immune response to radiotherapy
Abstract
Radiotherapy (RT) is considered a standard cancer treatment that directly kills tumor cells and promotes a systemic immune response. However, RT may also lead to tumor hypoxia, which further inhibits the antigen-presenting function of dendritic cells (DCs) and thereby weakens the systemic anti-tumor immune response induced by radiotherapy. In this study, the oxygen-loaded in situ gels carrying bacterial outer membrane (MOGel) were synthesized. As the gels slowly degraded, oxygen was gradually released to alleviate tumor hypoxia. The released bacterial outer membrane (OM) continuously activated DCs, enhancing their antigen-presenting capability. The results demonstrated that MOGel combined with RT induced the strongest tumor cell apoptosis in vitro and achieved a 80% tumor suppression rate in a colon cancer orthotopic model. Importantly, MOGel+RT induced an enhanced abscopal effect, and hypoxia and enhanced DCs activation contributed to the systemic immune response. Thus, OM-based oxygen gels may offer a novel strategy for enhancing the systemic immune response to RT.
Read More
Orthogonally Engineered Bacteria Capture Metabolically Labeled Tumor Antigens to Improve the Systemic Immune Response in Irradiated Tumors
Abstract
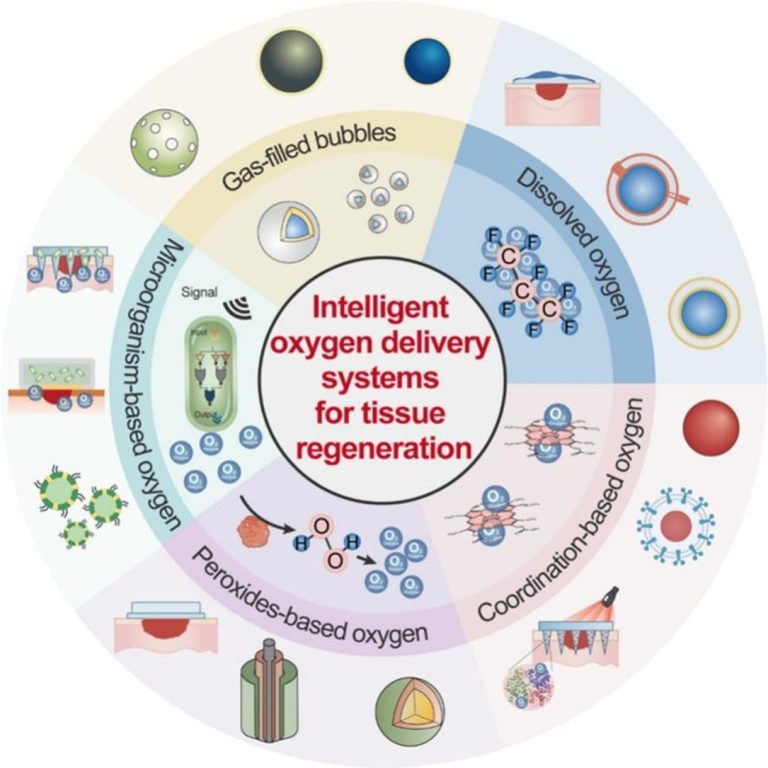
Oxygen plays a critical regulatory role in tissue repair and regeneration. However, in the microenvironment of tissues with vascular damage, hypoxia is commonly present. This not only suppresses cell proliferation and differentiation but also delays angiogenesis and extracellular matrix reconstruction, ultimately hindering the tissue regeneration process. Therefore, developing oxygen delivery strategies that can effectively enhance local oxygen levels has become one of the key approaches to promoting tissue regeneration. Traditional oxygen delivery strategies for tissue regeneration face several challenges, including inadequate sustained oxygen supply, poor targeting ability, and limited biocompatibility. To address these limitations, researchers have developed a variety of “intelligent oxygen delivery systems.” These systems can dynamically regulate oxygen release and achieve tissue-specific targeted delivery by responding to environmental or external stimuli, thereby significantly improving the precision and efficacy of oxygen therapy. This review systematically summarizes the biological functions of oxygen in tissue regeneration, with a particular focus on intelligent strategies for oxygen generation and supply developed in recent years. In addition, this review discusses the oxygen generation mechanisms, release kinetics, biocompatibility, application potential, and limitations of various oxygen delivery strategies. Finally, the review emphasizes that future designs of oxygen delivery systems should place greater emphasis on intelligent regulation, aiming to facilitate their clinical translation in tissue regeneration-related diseases such as chronic wounds, bone repair, and myocardial infarction.
Read More
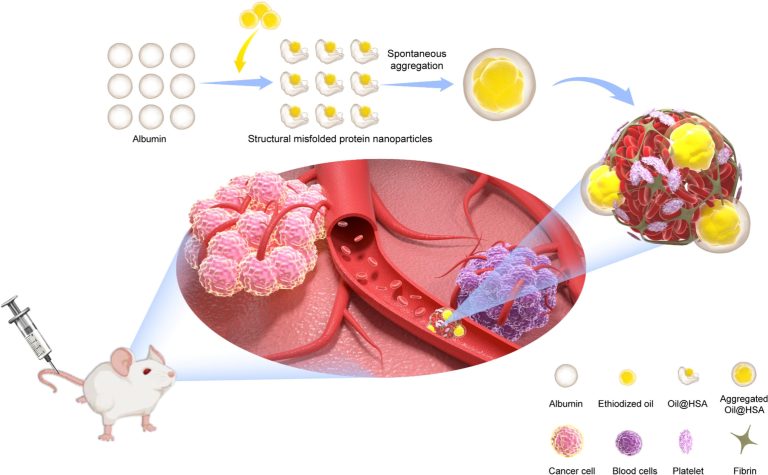
Protein-based embolic agent loaded with ethiodized oil for targeted tumor vascular embolization
Abstract
Tumor embolization therapy often involves complex procedures and poses challenges for physicians, especially with radiological imaging guidance. Inspired by physiological processes of misfolded protein aggregation and thrombus formation, we developed a protein-based embolic agent using ethiodized oil and human serum albumin for targeted tumor embolization. By occupying the hydrophobic cavity of albumin and disrupting its refolding process, these ethiodized oil bound human serum albumin nanoparticles (Oil@HSA NPs) achieve spontaneous aggregation and size growth at high concentrations, a unique advantage over conventional phospholipid nanoparticles. When combined with bacterium-induced coagulation in tumor vessels, Oil@HSA NPs selectively accumulate at tumor sites, blocking blood flow and significantly inhibiting tumor growth. This study introduces a practical and innovative approach to tumor embolization, offering a streamlined and effective alternative for vascular targeting in cancer therapy.
Read More
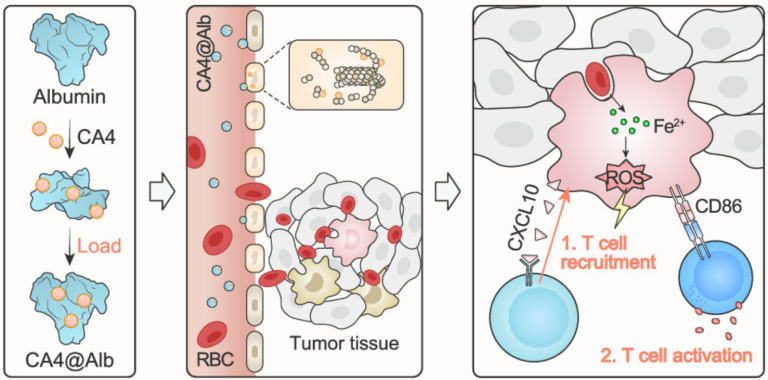
Combretastatin A4-Based Albumin Nanoparticles Remodeling the Tumor Immune Microenvironment to Enhance T Cell Immunotherapy in Colon Cancer
Abstract
The immunosuppressive tumor microenvironment formed by many solid tumors, particularly colon cancer, suppresses innate immune molecule expression and consequently limits T cell infiltration. Microtubule inhibitors were originally developed to eliminate tumors by inducing mitotic arrest. However, recent studies have shown that these inhibitors can also disrupt the microtubule dynamics and enhance the efficacy of immunotherapies. These findings highlight the target microtubule as a promising strategy for immune modulation. In this study, we investigated the use of Combretastatin A4 (CA4), a hydrophobic microtubule inhibitor that targets tumor vascular endothelial cells. To improve its solubility and delivery efficiency, CA4 was encapsulated in human serum albumin to form CA4@Alb. This formulation effectively inhibited microtubules in tumor endothelial cells, resulting in the promoted infiltration of erythrocytes into the tumor microenvironment. These erythrocytes were subsequently phagocytosed by intratumoral macrophages, leading to their pro-inflammatory activation. Notably, erythrophagocytic macrophages upregulated innate immune molecules, including chemokine CXCL10 and costimulatory molecule CD86, and enhanced T cell infiltration and activation. As a result, CA4@Alb significantly improved the responsiveness to T cell-based immunotherapies. Overall, our findings indicate that CA4@Alb effectively reprograms the immunosuppressive microenvironment of colon cancer and holds promising translational potential for enhancing immunotherapy efficacy.
Read More
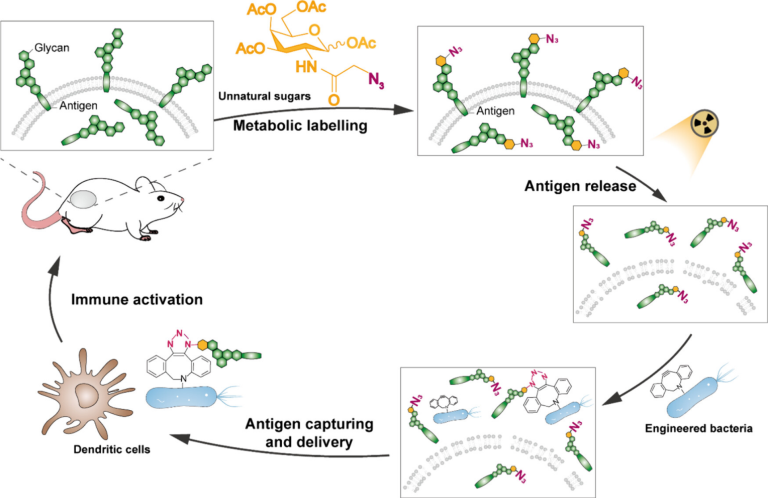
Orthogonally Engineered Bacteria Capture Metabolically Labeled Tumor Antigens to Improve the Systemic Immune Response in Irradiated Tumors
Abstract
In situ vaccination is considered a promising cancer immunotherapy strategy to elicit a tumor-specific T cell response. Live bacteria effectively enhanced the immune response in irradiated tumors as it can activate multiple immune cells. However, the adaptive immune response remains low since bacteria lack the efficient delivery of antigen to dendritic cells (DCs). Here, we show that tumor antigens can be metabolically labeled with azido groups in situ, allowing for their specific capture by orthogonally engineered Salmonella via bioorthogonal chemistry. Subsequently, these antigens are efficiently delivered to DCs through the active movement of the bacteria. Intratumorally injected engineered bacteria captured the labeled antigens and improved their presentation by DCs. This increased the proportion of antigen-specific CD8+ T cells in tumors, further resulting in systemic antitumor effects in the bilateral melanoma mouse model. The antitumor effects were abrogated in Batf3–/– mice or after CD8+ T cell depletion, indicating that systemic antitumor effects were dependent on adaptive immune responses. Overall, our work presents a strategy combining bacterial engineering and antigen labeling, which may guide the development of in situ vaccines in the future.
Read More
- Xia W, Feng Z, Wang Y, Lei R, Zhou Y, Zhuo Y, Xie R, Dong H, Zhao X, Guan X, Wu J. Orthogonally Engineered Bacteria Capture Metabolically Labeled Tumor Antigens to Improve the Systemic Immune Response in Irradiated Tumors. ACS Nano. 2025 Feb 11;19(5):5376-5391. doi: 10.1021/acsnano.4c13320. Epub 2025 Jan 31. PMID: 39889238.
> 2024
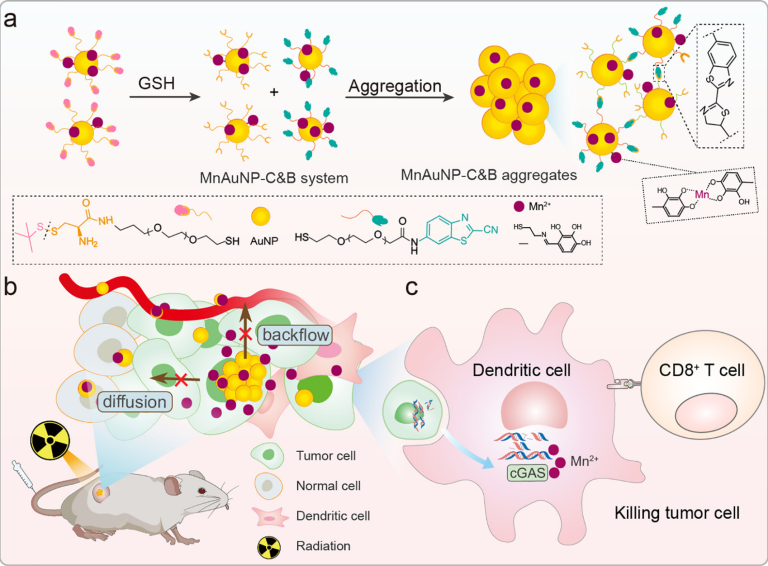
In Situ Aggregated Nanomanganese Enhances Radiation-Induced Antitumor Immunity
Abstract
Radiosensitizers play a pivotal role in enhancing radiotherapy (RT). One of the challenges in RT is the limited accumulation of nanoradiosensitizers and the difficulty in activating antitumor immunity. Herein, a smart strategy was used to achieve in situ aggregation of nanomanganese adjuvants (MnAuNP-C&B) to enhance RT-induced antitumor immunity. The aggregated MnAuNP-C&B system overcomes the shortcomings of small-sized nanoparticles that easily flow back into blood vessels and diffuse into surrounding tissues, and it also prolongs the retention time of nanomanganese within cancer cells and tumors. The MnAuNP-C&B system significantly enhances the radiosensitization effect in RT. Additionally, the pH-responsive disassembly of MnAuNP-C&B triggers the release of Mn2+, further promoting RT-induced activation of the STING pathway and eliciting robust antitumor immunity. Overall, our study presents a smart strategy wherein in situ aggregation of nanomanganese effectively inhibits tumor growth through radiosensitization and the activation of antitumor immunity.
Read More
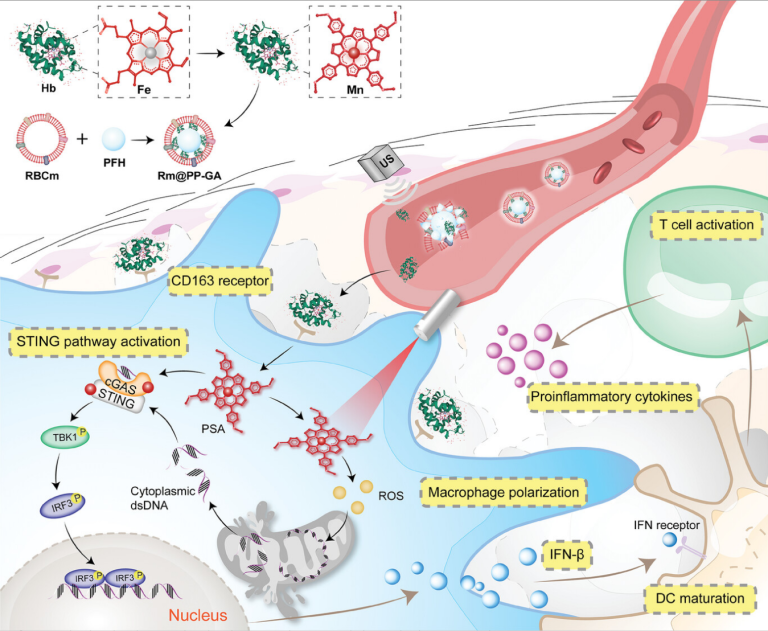
Novel Photo-STING Agonists Delivered by Erythrocyte Efferocytosis-Mimicking Pattern to Repolarize Tumor-Associated Macrophages for Boosting Anticancer Immunotherapy
Abstract
Immunotherapy has emerged as a highly effective therapeutic strategy for cancer treatment. Cyclic guanosine monophosphate-adenosine monophosphate synthase (cGAS)-stimulator of interferon gene (STING) pathway activation facilitates tumor-associated macrophage (TAM) polarization toward M1 phenotype, and Mn2+ are effective agents for this pathway activation. However, the high in vivo degradation rate and toxicity of Mn2+ hamper clinical application of immunotherapy. Here, this work has newly synthesized and screened manganese porphyrins for Mn2+ transport, referred to as photo-STING agonists (PSAs), and further encapsulate them into core-shell nanoparticles named Rm@PP-GA with dual specificity for tumor tissue and TAMs. Not only do PSAs achieve higher Mn2+ delivery efficiency compared to Mn2+, but they also generate reactive oxygen species under light exposure, promoting mitochondrial DNA release for cGAS-STING pathway activation. In Rm@PP-GA, globin and red blood cell membranes (Rm) are used for erythrocyte efferocytosis-mimicking delivery. Rm can effectively prolong the in vivo circulation period while globin enables PSAs to be taken up by TAMs via CD163 receptors. After Rm rupture mediated by perfluorohexane in nanoparticles under ultrasonication, drugs are specifically released for TAM repolarization. Further, dendritic cells mature, as well as T lymphocyte infiltrate, both of which favor tumor eradication. Therefore, cancer immunotherapy is optimized by novel PSAs delivered by erythrocyte efferocytosis-mimicking delivery pattern.
Read More
- Li Z, Li X, Lu Y, Zhu X, Zheng W, Chen K, Wang X, Wang T, Guan W, Su Z, Liu S, Wu J. Novel Photo-STING Agonists Delivered by Erythrocyte Efferocytosis-Mimicking Pattern to Repolarize Tumor-Associated Macrophages for Boosting Anticancer Immunotherapy. Adv Mater. 2024 Nov;36(47):e2410937. doi: 10.1002/adma.202410937. Epub 2024 Oct 8. PMID: 39380354.
- Xu J, Wang C, Zhang L, Zhao C, Zhao X, Wu J. In Situ Aggregated Nanomanganese Enhances Radiation-Induced Antitumor Immunity. ACS Appl Mater Interfaces. 2024 Jul 10;16(27):34450-34466. doi: 10.1021/acsami.4c03838. Epub 2024 Jun 28. PMID: 38941284.
- Gu L, Zhao C, Wang Y, Wang C, Yin X, Ye Q, Liu Y, Zou X, Wang L, Zhuge Y, Wu J, Zhang F. Senescence of Hepatic Stellate Cells by Specific Delivery of Manganese for Limiting Liver Fibrosis. Nano Lett. 2024 Jan 31;24(4):1062-1073. doi: 10.1021/acs.nanolett.3c03689. Epub 2024 Jan 2. PMID: 38164915; PMCID: PMC10836362.
- Ye Q, Zheng D, Chen K, Xu H, Yang Z, Wen J, Hu Y, Wu J. Phase-Change Based Oxygen Carriers Improve Acute Cerebral Hypoxia. Small. 2024 Jun;20(23):e2309180. doi: 10.1002/smll.202309180. Epub 2023 Dec 26. PMID: 38148304.
- Li Z, Li X, Lu Y, Zhu X, Zheng W, Chen K, Liu S, Wu J, Guan W. Improved Photodynamic Therapy Based on Glutaminase Blockage via Tumor Membrane Coated CB-839/IR-780 Nanoparticles. Small. 2024 Mar;20(10):e2305174. doi: 10.1002/smll.202305174. Epub 2023 Oct 24. PMID: 37875654.
> 2023
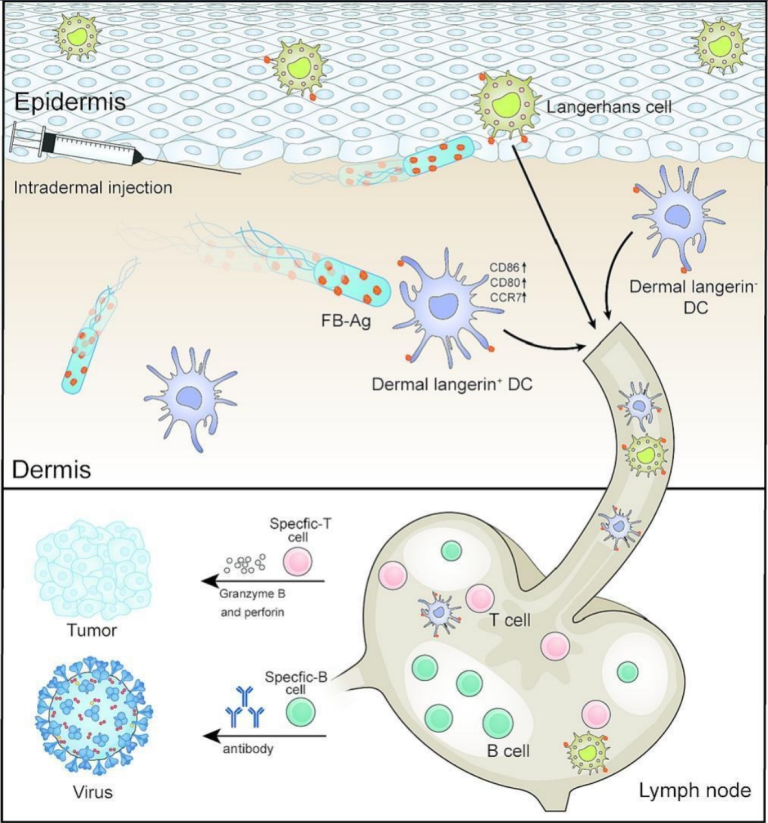
Antigen-loaded flagellate bacteria for enhanced adaptive immune response by intradermal injection
Abstract
Since the skin limits the distribution of intradermal vaccines, a large number of dendritic cells in the skin cannot be fully utilized to elicit a more effective immune response. Here, we loaded the antigen to the surface of the flagellate bacteria that was modified by cationic polymer, thus creating antigen-loaded flagellate bacteria (denoted as ‘FB-Ag’) to overcome the skin barrier and perform the active delivery of antigen in the skin. The FB-Ag showed fast speed (∼0.2 μm s−1) and strong dendritic cell activation capabilities in the skin model in vitro. In vivo, the FB-Ag promoted the spread of antigen in the skin through active movement, increased the contact between Intradermal dendritic cells and antigen, and effectively activated the internal dendritic cells in the skin. In a mouse of pulmonary metastatic melanoma and in mice bearing subcutaneous melanoma tumor, the FB-Ag effectively increased antigen-specific therapeutic efficacy and produced long-lasting immune memory. More importantly, the FB-Ag also enhanced the level of COVID-19 specific antibodies in the serum and the number of memory B cells in the spleen of mice. The movement of antigen-loaded flagellate bacteria to overcome intradermal constraints may enhance the activation of intradermal dendritic cells, providing new ideas for developing intradermal vaccines.
Read More
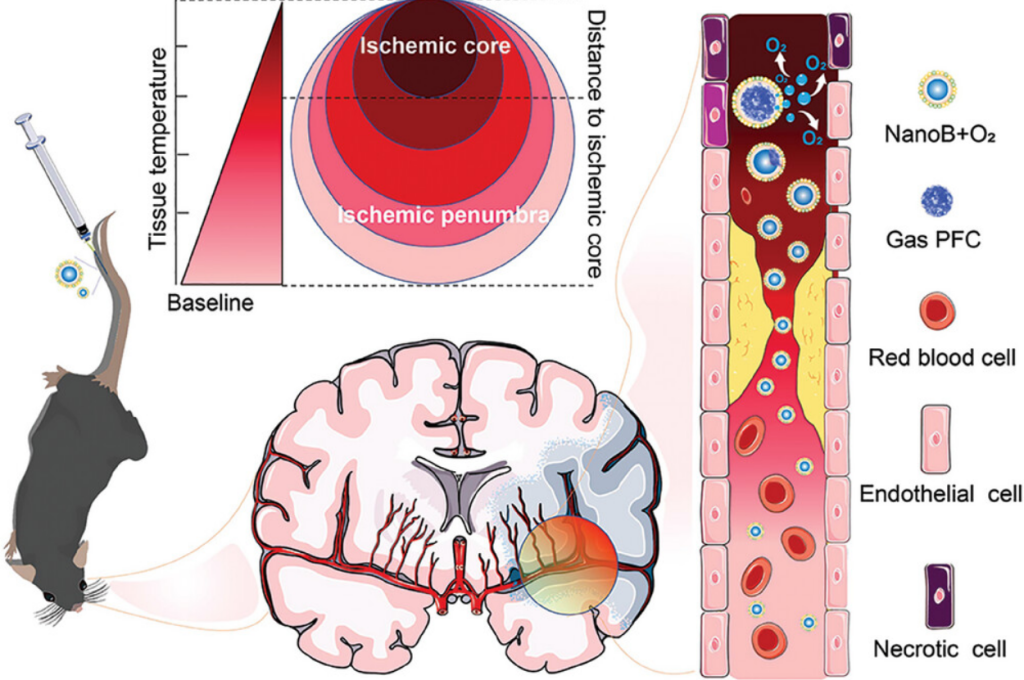
Phase-Change Based Oxygen Carriers Improve Acute Cerebral Hypoxia
Abstract
Stroke is the second leading cause of death worldwide, and hypoxia is a major crisis of the brain after stroke. Therefore, providing oxygen to the brain microenvironment can effectively protect neurons from damage caused by cerebral hypoxia. However, there is a lack of timely and effective means of oxygen delivery clinically to the brain for acute cerebral hypoxia. Here, a phase-change based nano oxygen carrier is reported, which can undergo a phase change in response to increasing temperature in the brain, leading to oxygen release. The nano oxygen carrier demonstrate intracerebral oxygen delivery capacity and is able to release oxygen in the hypoxic and inflammatory region of the brain. In the acute ischemic stroke mouse model, the nano oxygen carrier can effectively reduce the area of cerebral infarction and decrease the level of inflammation triggered by cerebral hypoxia. By taking advantage of the increase in temperature during cerebral hypoxia, phase-change oxygen carrier proposes a new intracerebral oxygen delivery strategy for reducing acute cerebral hypoxia.
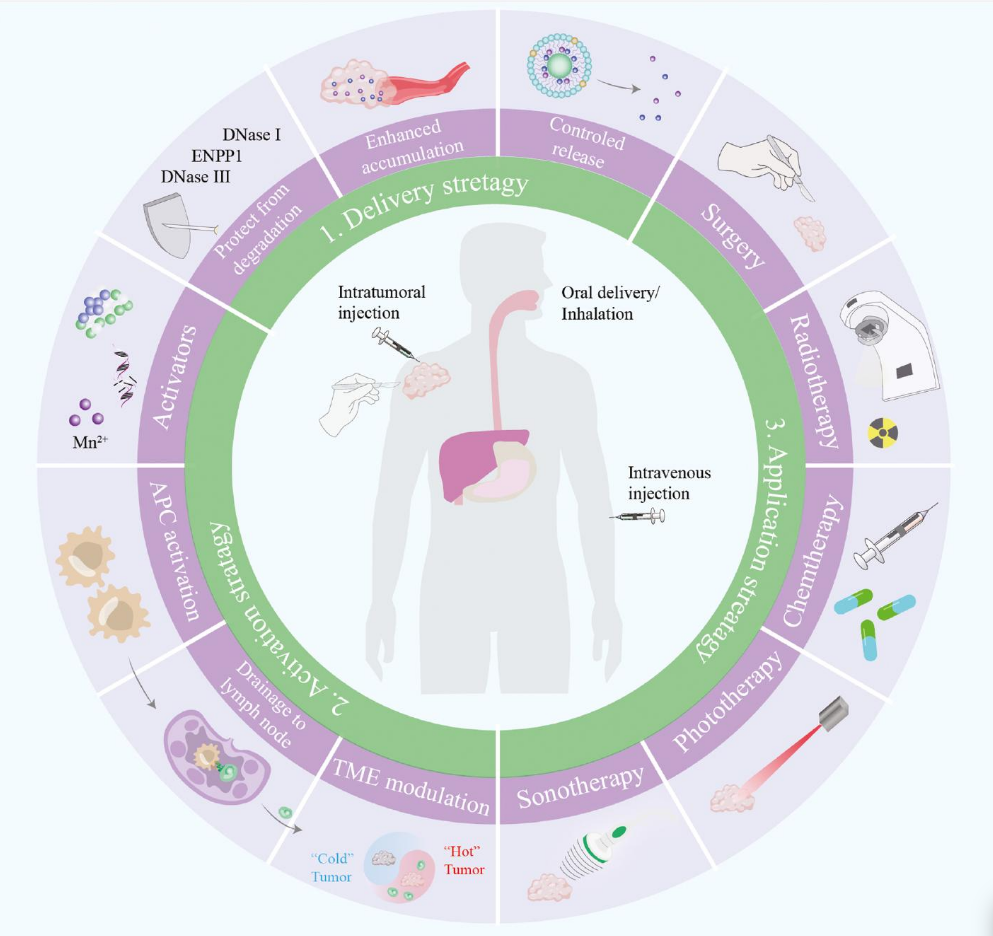
Novel emerging nano-assisted anti-cancer strategies based on the STING pathway
Abstract
Activation of simulator of interferon genes (STING), which induces the production of proinflammatory factors and immune effector cell activation, is considered a promising strategy for enhanced anti-cancer intervention. However, several obstacles prevent STING signaling in solid tumors, such as delivered molecules’ rapid degradation, restriction to tumor sites, insufficient intracellular concentrations, and low responsivity. Well-designed, multifunctional nano-formulations have emerged as optimized platforms for STING activation. Recently, a variety of nano-formulations have been developed and used in STING activation, thus facilitating immunotherapy in preclinical and clinical stages. Herein, we summarize recent advances in nanotechnology-based delivery, activation, and application strategies, which have advanced various aspects of immunotherapy. Novel STING agonists and their mechanisms in STING-activation-mediated tumor interventions are highlighted herein, to provide a comprehensive overview and discuss future directions for boosting immunotherapy via STING regulation.
- Zhiyan Li, Xianghui Li, Yanjun Lu, Xudong Zhu, Wenxuan Zheng, Kai Chen, Xingzhou Wang, Tao Wang, Wenxian Guan, Zhi Su*, Song Liu*, Jinhui Wu*,Novel Photo‐STING Agonists Delivered by Erythrocyte Efferocytosis‐Mimicking Pattern to Repolarize Tumor‐Associated Macrophages for Boosting Anticancer Immunotherapy。Advanced materials,2024 Oct 08: 2410937
- Xu Haiheng; Xiong Shuqin; Chen Yiyun; Ye Qingsong; Guan Nan; Hu Yiqiao; Wu Jinhui*. Flagella of tumor-targeting bacteria trigger local hemorrhage to reprogram tumor-associated macrophages for improved antitumor therapy. Advanced materials, 2023 Jun 13: e2303357.
- Zai Wenjing; Yuan Yunong; Kang Lin; Xu Jialong; Hu Yiqiao; Kang Lifeng; Wu Jinhui*. Oxygen penetration through full-thickness skin by oxygen-releasing sutures for skin graft transplantation. Engineering. 2023 May 29: 10.1016/j.eng.2023.05.006.
- Yang Liu, Yuchen Wang, Chao Wang, Tiejun Dong, Haiheng Xu, Yunfei Guo, Xiaozhi Zhao*, Yiqiao Hu*, Jinhui Wu*, Hijacking Self-Assembly to Establish Intracellular Functional Nanoparticles. Adv Sci. 2022 Sep 8;e2203027
- Huanhuan Chen, Yunfei Guo, Zhewei Zhang, Wenxuan Mao, Chenying Shen, Wei Xiong, Yingfang Yao, Xiaozhi Zhao, Yiqiao Hu, Zhigang Zou, and Jinhui Wu*. Symbiotic Algae–Bacteria Dressing for Producing Hydrogen to Accelerate Diabetic Wound Healing. Nano Letter, 2021, 20 December
- Wenjing Zai, Lin Kang, Tiejun Dong, Haoran Wang, Lining Yin, Shaoju Gan, Wenjia Lai, Yibing Ding, Yiqiao Hu, Jinhui Wu*.E. coli Membrane Vesicles as Catalase Carrier for Long Term Tumor Hypoxia Relief to Enhance Radiotherapy. ACS Nano, 2021, 15, 9, 15381–15394.
- Wenguang Wang, Haiheng Xu, Qingsong Ye, Feng Tao, Ian Wheeldon, Ahu Yuan, Yiqiao Hu, Jinhui Wu, Systemic immune responses to irradiated tumours via the transport of antigens to the tumour periphery by injected flagellate bacteria. Nature Biomedical Engineering. 2021. (Highlight in Nature Biomedical Engineering)
- Chao Wang, Zhaoyi Sun, Chenxuan Zhao, Zhewei Zhang, Haoran Wang, Yang Liu, Yunfei Guo, Bingtao Zhang, Lihong Gu, Yue Yu, Yiqiao Hu, Jinhui Wu*. Maintaining manganese in tumor to activate cGAS-STING pathway evokes a robust abscopal anti-tumor effect. Journal of Controlled Release, 2021, 331,480
- Huanhuan Chen, Yuhao Cheng, Jingrun Tian, Peizheng Yang, Xuerao Zhang, Yunhao Chen, Yiqiao Hu*, Jinhui Wu*. Dissolved oxygen from microalgae-gel patch promotes chronic wound healing in diabetes. Science Advance, 2020, 6(20), eaba4311
- Zaigang Zhou, Baoli Zhang, Wenjing Zai, Lin Kang, Ahu Yuan, Yiqiao Hu*, Jinhui Wu*, Perfluorocarbon nanoparticle-mediated platelet inhibition promotes intratumoral infiltration of T cells and boosts immunotherapy. PNAS, 2019 May 29. pii: 201901987.
- Wenguang Wang, Yuhao Cheng, Peng Yu, Haoran Wang, Yue Zhang, Haiheng Xu, Qingsong Ye, Ahu Yuan, Yiqiao Hu, Jinhui Wu*, Perfluorocarbon regulates the intratumoural environment to enhance hypoxia-based agent efficacy. Nature communications. 2019, 10:1580.